Adhesion Force
One day, a clerk in the shop tried to tag labels on merchandise. Have you ever considered why the labels can be affixed well on the goods?
One of the important secrets in the glue is Adhesion Force.
What does the adhesion force of the glue mean? Adhesion force is the attraction strength between adhesive and substrate. The bond formed between the adhesive and the surface is known as adhesion. However, the adhesion force is not the only factor important to creating an effective bond. Even when using the world’s strongest adhesive, a bond will fail if the adhesive does not fit and can not create a bond to the surface of the substrate.
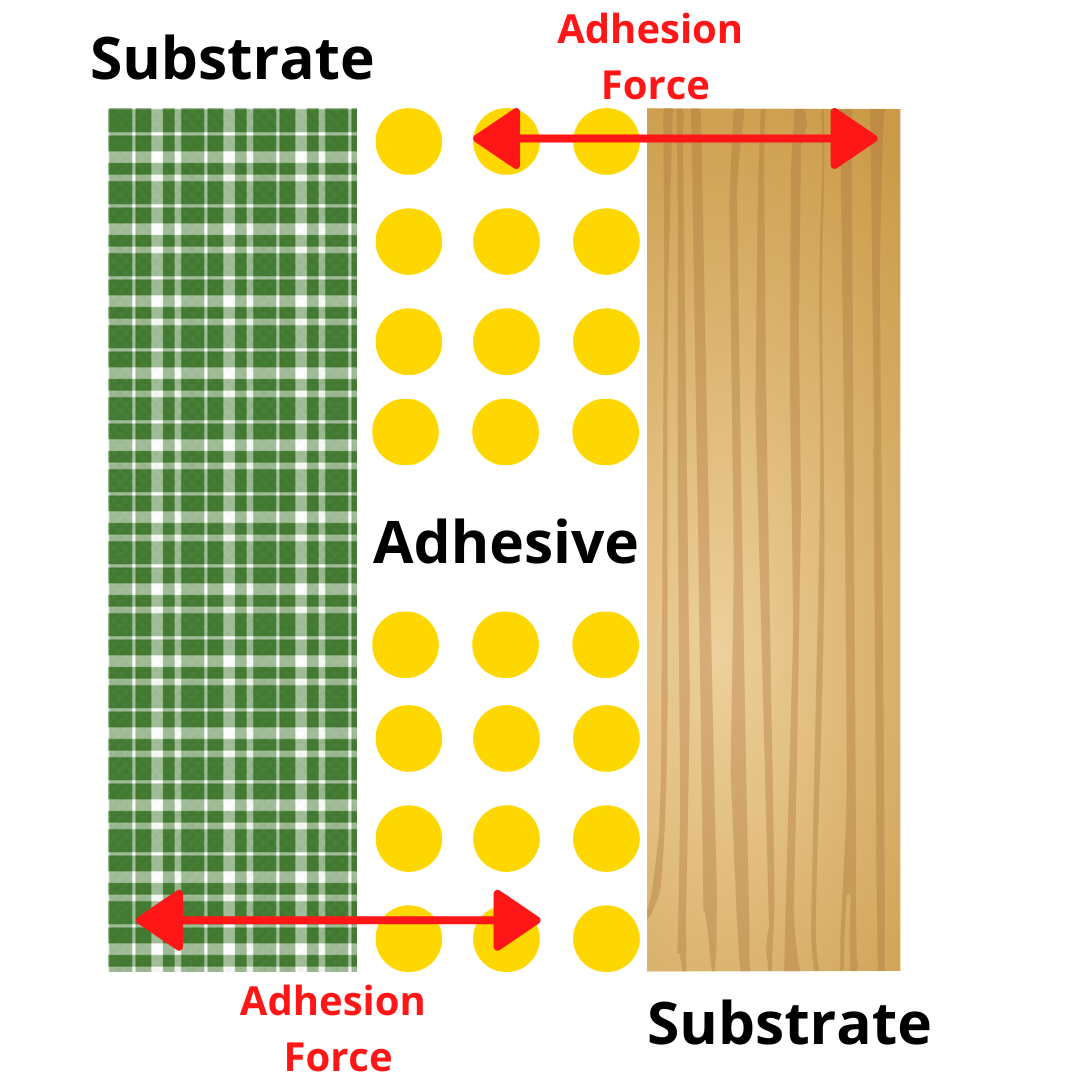
As a result, the adhesion force of the glue is also influenced by the substrate’s surface energy and wetting ability.
Surface energy is a physical property of the surface of a material that determines whether an adhesive will make intimate contact. On a material with high surface energy, the liquid or adhesive will wet out or spread out on the surface evenly; on a material with low surface energy, the liquid or adhesive will resist flowing and bead up. An adhesive must wet out the substrate to provide a bond.
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. Wetting is an important factor in the bonding or adherence of two materials.
Another thing that affects the adhesive contact’s effectiveness is its shape. Adhesive contacts of complex type tend to separate at the “edges” of the contact zone.
For more knowledge about adhesion please click here.
Want to know why adhesive is sticky? please click here.
